The Future of Healthcare: Understanding Remote Patient Monitoring in the United States
Remote Patient Monitoring (RPM) is revolutionizing the way healthcare is delivered in the United States. This innovative approach leverages smart medical devices and cloud-based platforms to track patients’ health data in real time, outside of traditional clinical settings. As the demand for more efficient, patient-centered care grows, RPM has emerged as a critical component of modern healthcare systems. From chronic disease management to post-surgical recovery, RPM is transforming how providers deliver care and how patients engage with their health.
What Is Remote Patient Monitoring?
Remote Patient Monitoring (RPM) is a form of telehealth that utilizes technology to collect health data from patients in one location and send it to healthcare providers in another. The goal of RPM is to improve the value of care by allowing monitoring in real time or remotely, outside of the clinical setting, most often in the patient’s home. RPM works to collect metrics of multiple physiological variables using digital FDA-approved devices that monitor vitals, such as:
- Blood pressure
- Heart rate
- Glucose levels
- Blood oxygen saturation (SpO₂)
- Weight
- Temperature
- Respiratory rate
The patient’s health data is transmitted to a healthcare team through a secure cloud-based platform. Multiple providers can work in conjunction with algorithms using artificial intelligence (AI) and machine learning, which are trained to flag anomalies and create actionable insights.
Key Features of RPM
- Continuous Monitoring: Unlike assessments that occur only during a clinic visit, RPM is continuous, daily, even hourly.
- Preventive Strategy: RPM identifies early signs of deterioration, allowing for intervention before hospital visits are required.
- Patient Involvement: Patients are involved in their care by having their data monitored.
These features contribute to improved clinical outcomes, better care satisfaction, and a lighter load on already overwhelmed health systems.
The Purpose of Remote Patient Monitoring
The key strength of Remote Patient Monitoring is that it offers an efficient way to provide oversight of a patient’s health condition while narrowing the gap between in-person visits and self-care. RPM is especially beneficial for chronic disease management, post-acute care, and managing higher-risk populations.
Benefits of RPM
- Chronic Disease Management: RPM is effective for chronic diseases such as hypertension, diabetes, heart failure, COPD, and other chronic illnesses. Advances in technology now enable clinicians to make alterations to treatment plans in real time and adjust medications before complications arise.
- Prevention of Hospital Re-admissions: RPM allows clinicians to intervene more quickly to prevent a patient from returning to the hospital. This is the most common quality metric and is valuable for clinicians to consider during the 30 days after discharge, since hospitals are penalized with decreased Medicare reimbursement via CMS for re-admissions.
- Increasing Preventive Care: RPM allows clinicians to intervene when early warning signs such as high glucose levels or abnormal heart rhythms appear. This process reduces disease prevalence and even saves money in the healthcare system.
- Patients Are No Longer Passive Participants in Their Care: RPM encourages patients to be more engaged, thus promoting self-awareness, medication adherence, and health literacy.
- Advocating for Value-Based Care: RPM aligns with value-based care models that reward better outcomes, not the sheer volume of services. RPM can provide measurable metrics to demonstrate improved quality, population health management, and cost containment.
- Providing Equitable Access to Care: For patients in rural or underserved locations, RPM provides access to near-continuous care without the burden of traveling long distances. This addresses inequities and promotes the improvement of population health metrics.
Components of Remote Patient Monitoring
To effectively implement RPM, several components must be in place:
1. Remote Monitoring Devices
These are the first point of contact with the patient’s data. Devices vary depending on the condition and target of monitoring. Examples include:
- Blood pressure monitors
- Glucometers
- Pulse oximeters
- Smart scales
- ECG devices
- Smart thermometers
All devices used need to be FDA-approved and able to securely transmit the data.
2. Connectivity and Communication Networks
RPM devices can send patient data via:
- Bluetooth
- Cellular networks
- Wi-Fi
- IoT-enabled devices
To maintain security, data is typically encrypted in a manner consistent with HIPAA standards.
3. Patient Portal or Mobile Application
Patients are typically exposed to RPM via an app or portal. The interface allows:
- Visualizing their health trends
- Messaging or alerts
- Completion of health questionnaires
- Scheduling reminders for medication and tests
Apps will also frequently offer educational materials, chat support, and feedback options.
4. Clinical Dashboard for Providers
Clinicians have a single dashboard for reviewing:
- Patient trends
- AI-generated alerts
- Adjustments to care plans
- Patient engagement
Modern dashboards allow customization, prioritization of high-risk patients, and seamless access from Electronic Health Records (EHRs).
5. Data Analytics & Decision Support
AI & ML algorithms analyze large quantities of incoming data to reveal trends, flag anomalies, and provide predictive suggestions. They can do things like:
- Determine medication side effects
- Indicate emergencies
- Suggest adjustments to care plans
6. Electronic Health Records Integration
Integrated Electronic Health Records allow for RPM data to be included in the patient’s longitudinal health record. This allows better care coordination and compliance with documentation standards.
7. Support and Training Infrastructure
RPM onboarding and support are key factors for successful RPM implementation. Teaching patients how to use devices and read their data is part of the connectivity. It is important for patients to know how to respond to alerts. Likewise, providers must be onboarded to their dashboards, billing, and compliance.
Types of RPM Devices That Simplify Patient Monitoring
There are a variety of different RPM devices to collect patient data. For patient safety, accuracy, and reimbursements, it is important RPM devices be cleared or approved by the FDA. Most 510(k) devices are cleared under the FDA’s 510(k) clearance process that denotes the device is substantially equivalent to a legally marketed device. This clearance also makes the device eligible for CMS reimbursement under RPM specific CPT codes. The most commonly used devices include:
| Device | Description |
|---|---|
| Blood Pressure Monitors | Used for managing hypertension and cardiovascular disease. |
| Glucometers | Essential to managing diabetes. CGM or Continuous Glucose Monitor provides real-time glucose levels. |
| Pulse Oximeters | Measure oxygen saturation. Useful in the management of COPD or during recovery from respiratory illnesses. |
| Smart Scales | Track fluid retention and weight changes. Useful in managing heart failure and obesity. |
| Wearable ECG Monitors | Continuously assess the electrical activity of the heart. Enable early detection of arrhythmias. |
| Thermometers | Useful to monitor infection and fluctuating body temperature post-op. |
| Spirometers | Used to monitor lung function in asthma and COPD patients. |
| Wearables (watches) | Monitor heart rate, activity, and sleep patterns. Enable both preventative and chronic care models. |
| Medication Adherence Tools | Smart dispensers or notification systems help reduce missed medication doses. |
| Multi-parameter Devices | Combine different sensors into one device. Useful for patients with complex medical conditions. |
Most of the devices listed above come complete with the ability to sync to smartphones via Bluetooth or cellular signals, graft patients onto EHRs, and supply patients with a mobile app to ensure data is easily transmitted and flowed between the patients and providers.
How Does RPM Work?
RPM platforms serve as a connection point between patients, clinicians, and relevant health data in real-time. These platforms utilize FDA-approved medical devices and software where clinicians can establish reporting on patients’ vital signs. Here’s a breakdown of the RPM process:
1. Patient Enrollment & Onboarding
The RPM journey begins with the identification of eligible patients, generally those managing a chronic illness, such as hypertension, diabetes, COPD, or those recently discharged from an inpatient hospital setting. RPM patients can be enrolled and onboarded from clinician referral, use of algorithms from population health, or during transitions of care.
2. Daily Vital Sign Collection
Once patients are enrolled and set up, they start to use their devices to monitor important vitals. The parameters include blood pressure, blood glucose, oxygen saturation (SpO₂), weight, heart rate, and sometimes, the respiratory rate or ECG rhythms. The ease of use is an important consideration. The devices are Bluetooth-enabled, directly connecting to an app or home gateway device automatically. Some also use cellular transmission built into each device, which eliminates the need for Wi-Fi access in the home and creates more access for at-home monitoring.
3. Secure Data Transmission & Storage
Data security is a foundation of all RPM platforms. Patient health information (PHI) needs to be encrypted in storage and in transfer to satisfy HIPAA guidelines. Once the data is captured via the RPM device, it transfers to secure networks in real time for storage. Each reading is automatically marked with the date and time, tagged, and laid out in an organized way.
4. Real-Time Alerts & Notifications
Arguably the most important aspect of RPM platforms is their ability to produce real-time alerts around clinical thresholds. These thresholds are either set or programmed based on clinical guidelines or customized for a certain patient. If a reading passes above threshold or drops below threshold, an automated alert is shared with the clinical care team. Patients may receive an automated app alert with self-care advice or prompts for follow-up.
5. Clinical Review & Interventions
While AI and automated alerts provide the core of the RPM platforms, there is still a necessary human role. Clinicians interact with an intuitive provider dashboard to see individual readings, track trends, and prioritize patients needing intervention. Clinicians are also able to send secure messages with the patient from the dashboard, change care plans, and schedule follow-ups.
Benefits of Remote Patient Monitoring
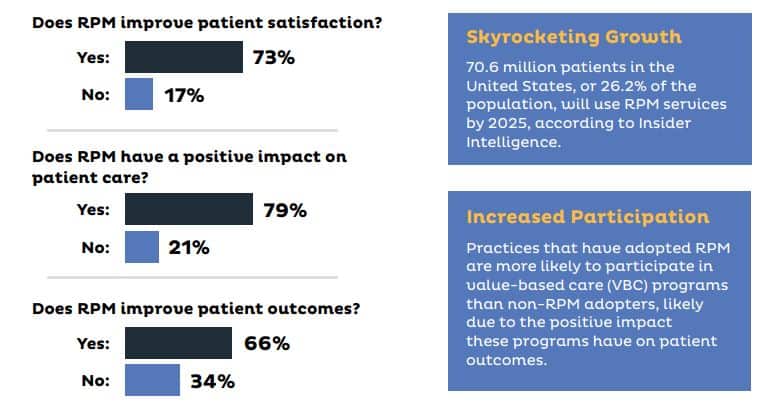
Top Benefits for Healthcare Providers
- Better Clinical Outcomes: 24/7 monitoring enables early intervention, personalized care plans, and real-time clinical decisions.
- Reduction of ER Utilization and Hospital Readmissions: RPM helps reduce readmissions, especially post-discharge, avoiding CMS penalties.
- Improved Workflow Efficiency: Automated alerts and data collection let care teams focus on critical interventions.
- New Billing Opportunities: Reimbursement available through CPT codes (99453, 99454, 99457, 99458) increases revenue potential.
- Aligned with Value-Based Care: Supports KPIs like reduced ER visits and better chronic disease management.
- Improved Patient Engagement and Adherence: Video guides and reminders enhance adherence to treatment plans.
- Real-Time Data Insights: Trends and predictive analytics support better diagnoses and proactive care.
- Expanded Reach and Scalability: Enables monitoring of more patients without requiring more staff, ideal for rural/underserved regions.
- Integration with EHRs: Seamless EHR integration improves data continuity and care coordination.
- Competitive Advantage: Positions providers as innovative leaders using tech-savvy care models.
Top Benefits for Patients
- Continuous Health Monitoring: Ongoing tracking of vitals offers peace of mind and clinical awareness.
- Earlier Detection of Health Issues: Enables early treatment through real-time alerts.
- Convenience of RPM: Patients can measure vitals from home, improving access for elderly/rural individuals.
- Focused Attention on Your Care: Personalized treatment plans improve outcomes and satisfaction.
- Better Health Literacy: Educational content helps patients understand and manage their health.
- Better Communication with Providers: Secure messaging and telehealth allow quicker, easier communication.
- Promotion of Healthy Behaviors: Daily monitoring encourages medication, diet, and exercise adherence.
- Decreased Hospitalizations and ER Visits: Fewer emergency situations due to proactive intervention.
- Cost Savings: Reduced need for ER visits and long hospital stays lowers costs.
- Improved Satisfaction and Trust: Patients feel more in control and connected with providers.
RPM vs. Other Remote Health Monitoring Programs
Faced with multiple remote health monitoring solutions, Remote Patient Monitoring (RPM) is the best choice among digital health solutions because of conclusive evidence of better health outcomes, a comprehensive reimbursement structure, and its capacity to engage care at scale with patient populations.
| Advantage Area | Remote Patient Monitoring (RPM) | Other Remote Health Monitoring Programs |
|---|---|---|
| Reimbursement Support | Reimbursement-Driven and Backed by CMS with established CPT codes. | Wellness apps or telehealth often lack long-term reimbursement models. |
| Clinical vs. Lifestyle Focus | Clinical Monitoring Focused – uses FDA-approved devices for data-driven decisions. | Often limited to lifestyle engagement via fitness trackers or wellness apps. |
| Chronic Care Coordination | Works in tandem with CCM for chronic care documentation and outcome improvement. | Limited capability to coordinate care or support chronic conditions. |
| Value-Based Care Alignment | High adoption among ACOs and Medicare Advantage for meeting quality metrics. | Less aligned with value-based care and performance tracking. |
| Real-Time Insights | Provides real-time, actionable data for proactive clinical interventions. | Typically provides delayed or aggregate data. |
| Regulatory Compliance | Meets FDA standards and clinical regulatory thresholds. | Less regulated; may not meet clinical-grade standards. |
| Patient Trust & Engagement | Device prescribed by a clinician with direct feedback builds trust and engagement. | Self-managed apps often suffer from low long-term engagement. |
| Population Health Enablement | Enables large-scale monitoring, risk stratification, and nursing alerts. | Limited in stratifying risks or reaching broad patient populations. |
| EHR Integration | Fully integrates with EHRs for seamless workflows, documentation, and billing. | Often operates separately without EMR integration. |
| Scalability | Cloud-based and scalable without major infrastructure investment. | Usually not scalable beyond basic app or device capabilities. |
Eligibility Criteria for RPM
Patient Eligibility
- Must be a Medicare beneficiary or a patient under some private insurances.
- Patient or legal guardian must consent to RPM services (verbal or written consent is acceptable, but must be documented in the medical record).
- RPM services must be ordered by a qualified healthcare provider and be part of the patient’s treatment plan.
- RPM services must be medically necessary.
Provider Eligibility
- Only Medicare-eligible providers who are authorized to bill Evaluation and Management (E/M) services can order and bill for RPM, including Physicians (MD/DO), Nurse Practitioners (NPs), Physician Assistants (PAs), Clinical Nurse Specialists (CNSs), and Certified Nurse Midwives (CNMs).
- RPM services can be delivered under general supervision, meaning the supervising provider does not need to be physically present. Clinical staff (e.g., nurses, MAs) may help monitor and communicate with patients as long as a qualified provider oversees and bills for the service.
- Providers must be licensed in the state where the patient resides.
- Services must fall within the provider’s scope of practice as defined by state law.
- Telehealth laws may affect cross-state RPM provision.
- Providers must use FDA-defined medical devices capable of:
- Automatic, electronic transmission of patient data
- Secure, HIPAA-compliant RPM software
- Must have access to a HIPAA-compliant RPM platform or dashboard to store, analyze, and respond to incoming patient data.
The Timeline for Remote Patient Monitoring Services
Implementing RPM involves a structured timeline to ensure success:
Phase 1: Assessment & Eligibility Review
- Timeframe: Week 1
- Description: Providers must determine the patient’s eligibility before they can offer RPM services.
- Key Activities:
- Identify chronic/acute conditions
- Verify insurance eligibility (Medicare/private)
- Obtain patient consent
- Select RPM technology based on care needs
Phase 2: Device Selection & Enrollment
- Timeframe: Week 1–2
- Description: The provider will choose a suitable RPM device once the patient’s eligibility status has been established.
- Key Activities:
- Order FDA-cleared RPM device (e.g., BP monitor, glucometer)
- Enroll patient on platform (e.g., HealthArc)
- Set up patient profile and data transfer
- Train patient on device use
Phase 3: Initial Data Collection & Calibration
- Timeframe: Weeks 2–3
- Description: Begin capturing data and ensuring stable transmission.
- Key Activities:
- Confirm accurate device pairing
- Resolve connectivity issues
- Educate patient for regular usage (≥16 days/month)
- Set clinical thresholds for alerts
Phase 4: Active Monitoring and Clinical Review
- Timeframe: Ongoing from Month 1
- Description: Continuous review of health data and clinical response.
- Key Activities:
- Daily review of patient data
- Trigger alerts for abnormal values
- Schedule/document telehealth check-ins
- Record clinical interventions and notes
Phase 5: Monthly Reporting & Billing
- Timeframe: End of Each Month
- Description: Validate compliance and submit reimbursement claims.
- Key Activities:
- Verify 16-day minimum data for CPT 99454
- Compile monthly report
- Submit billing claims (99453, 99454, 99457, 99458)
- Update medical records and outcomes
Phase 6: Long-Term Continuity and Reassessment
- Timeframe: Every 3–6 Months
- Description: Reevaluate RPM effectiveness and adjust care plans.
- Key Activities:
- Review patient status and RPM necessity
- Upgrade/replace devices if needed
- Confirm ongoing patient engagement
- Modify care plan based on progress
The Regulatory Environment for RPM
The U.S. Food and Drug Administration (FDA) plays a crucial role in the design, approval, and post-marketing surveillance of RPM devices. Adhering to FDA regulations is not only an obligation but also a commitment to patient safety, clinical accuracy, and the ability to bill for services.
Device Classification and FDA Clearance
RPM devices can be classified into several categories of medical devices by the FDA, all of which are classified in class I, class II, or class III. Many RPM devices, such as blood pressure cuffs, glucose meters, wearable ECG monitors, are classified as class II because they require a premarket notification (510(k) clearance) that demonstrates they are “substantially equivalent” to a previously legally marketed device and that have certain safety and performance standards.
Cybersecurity Standards
Given that many RPM systems are transporting sensitive health data to cloud platforms or mobile applications, cybersecurity standards have become a regulatory priority. The FDA requires that manufacturers include cybersecurity in the design and development phases of the system to the point where the device includes encryption, secure firmware update processes, user authentication, and measures to prevent unauthorized access to personal health information and manipulation of the devices.
Quality System Regulation (QSR)
The FDA implements the Quality System Regulation (21 CFR Part 820), which defines current Good Manufacturing Practices (cGMP) for medical devices. These manufacturers of RPM devices must establish and maintain a comprehensive quality system that controls the design and testing of the product, the manufacturing process, and corrective action, to ensure that every time they produce the device it is consistently reliable and safe.
Post-Market Surveillance and Adverse Event Reporting
The RPM device will still go through continued regulatory scrutiny in the way of post-market surveillance protocols, even after obtaining FDA clearance. Manufacturers will be required to verify the device’s functionality, and if there were issues reported by users or adverse events associated with their device, they will have to communicate these to the FDA through the Medical Device Reporting (MDR) process.
Digital Health Software Precertification Program
To ensure that the rapid health technology digital ecosystem keeps evolving responsibly, the FDA created a Software Precertification Pilot Program, focused on the FDA’s Digital Health Innovation Action Plan. This program establishes a model relying on a company’s software development capabilities rather than reviewing individual products. The FDA has an interest in enabling manufacturer’s speed of entry into the markets with digital health tools, including RPM software, without sacrificing the safety for their consumers.
Reimbursement Considerations
From a provider’s standpoint, utilizing RPM devices that have been cleared by the FDA is a necessary precondition to cover eligible Medicare RPM billing codes (e.g., CPT 99453, 99454, 99457, and 99458). Most commercial payers have similar restrictions. Providers should document all RPM hardware and software utilized in the care of their patients, including FDA clearance.
Insurance Coverage for Remote Patient Monitoring (RPM)
Medicare Reimbursement for RPM
Medicare is a leader in encouraging RPM services, especially with the recent expansion of telehealth during the COVID-19 Public Health Emergency. Key Takeaways:
- Medicare Part B covers RPM services under CPT codes 99453, 99454, 99457, and 99458.
- RPM services must be ordered by a physician or qualified provider.
- The patient must have a chronic or acute condition that requires ongoing monitoring.
- Medicare typically reimburses 80% of the approved cost, and the patient is responsible for 20%, unless they have a supplemental insurance plan.
Medicaid Coverage
Varies by State. Medicaid coverage is up to the authority of each state. Many states followed Medicare’s lead, but each has created individualized guidelines. For example:
- California: Covers RPM for management of chronic conditions with certain requirements.
- Texas: Medicaid pays for devices and provides a monthly payment for monitoring.
- New York: Covers the use of RPM through fee-for-service and managed care.
Providers need to check their state’s Medicaid manual or contact the state Medicaid agency for specifics.
Private Insurance Coverage
Most private payers provide some form of coverage for RPM, especially in value-based contracting and employer-sponsored arrangements. Common coverage includes:
- Remote monitoring for chronic disease management (e.g., diabetes, hypertension)
- Bundled care packages where RPM is included in telehealth service delivery
- Preventive care and readmission reduction initiatives
Some of the key insurers providing RPM services include UnitedHealthcare, Blue Cross Blue Shield, Cigna, and Aetna.
Veterans Affairs (VA) Approaches to RPM
The VA is leading the charge in using RPM to manage chronic conditions in veterans via VA RPM Programs that provide:
- Free devices and data plans for veterans
- RPM services are available in VA hospitals and clinics
- Access to RPM telehealth nurses to assist with monitoring
Coverage Levels and Restrictions
Regardless of the payer, RPM services must meet specific coverage criteria to qualify for reimbursement. Common Requirements:
- Use of an FDA-cleared device
- Collection of data for a minimum of 16 days in a 30-day monitoring period
- Clinical review and patient interaction must be documented
- Monitoring must be part of a treatment plan
Common Limitations:
- The device must not be part of a larger bundled payment
- Non-FDA-cleared consumer devices may not qualify
- Provider failure to document will result in denied reimbursement
Device Coverage
The cost of RPM devices may be reimbursed by insurers in several ways:
- Rental – Devices can be rented to patients
- Ownership – Durable medical equipment (DME); devices are provided
- Software-as-a-Service (SaaS) – Device fees bundled as part of the RPM package
The device cost is typically included under CPT code 99454, which relates to the supply and transmission of the device.
Co-pays and Out-of-Pocket Costs
Patients, even if covered by insurance, may incur co-pays, deductibles, and other out-of-pocket costs, including:
- Medicare: 20% coinsurance unless covered by a Medigap plan
- Private Plans: Varies by plan and individual deductible; high-deductible plans may require payment upfront
- Medicaid: Low to no co-pays, depending on the state
Value-Based Care Models
RPM is frequently a part of value-based care contracts, where providers are compensated for quality outcomes. Some examples include:
- Reduced ER visits and hospital readmissions
- Improved adherence to treatment plans
- Increased patient satisfaction scores
Value-based care models often encourage payers to invest in RPM as a cost-saving and quality improvement measure.
Emerging Coverage Trends for 2025
Recent changes indicate a movement toward broader RPM insurance coverage. Some trends include:
- Increased support for mental health & behavior monitoring
- Expanded use in maternal and pediatric care
- Improved coverage for mobile app and wearable integration
The CMS Innovation Center is also testing new models to promote RPM use in primary care and underserved populations.
Confirming Successful Reimbursement
Healthcare providers can confirm RPM reimbursement eligibility if they:
- Verify insurance eligibility and coverage before enrollment
- Maintain proper documentation and patient consent
- Use certified RPM vendors
- Stay updated on payer billing requirements
With reimbursement for Remote Patient Monitoring expanding rapidly, there is support from Medicare, Medicaid, private insurers, and the VA, each providing varying levels of reimbursement to facilities and providers, and increasing support for patients.
RPM Billing Guidelines – 2025 Edition
Overview of RPM Billing
RPM billing follows the Medicare Physician Fee Schedule (MPFS) structure, and there are multiple Current Procedural Terminology (CPT) codes that dictate how and when these services can be billed, including device setup, patient monitoring, and time spent on data analysis. Private payers and Medicaid plans have been aligning their reimbursement structures with CMS policies.
Important Billing Guidelines
To bill for RPM, it must be established that:
- The patient has a chronic or acute condition that requires monitoring.
- The RPM device is FDA-cleared/approved and allows for automatic data upload.
- Data was collected for 16 or more days within a rolling 30-day billing window.
- The services were referred by a physician or qualified healthcare provider.
- The patient’s consent to monitor their data, and that it was clinically relevant, must be documented.
CPT Codes for RPM Billing
| CPT Code | Service Description |
|---|---|
| 99453 | Device setup and patient education |
| 99454 | Device supply and daily recording per patient |
| 99457 | First 20 minutes of interactive communication |
| 99458 | Additional 20-minute increments |
| 99091 | Collection and interpretation of physiologic data |
Standards of Compliance and Documentation
Providers must include the following in clinical documentation:
- Start and end date of monitoring
- Data trends, if clinically relevant
- Patient communications
- Completed patient consent forms and a signed physician order
Best Practices for Billing
- Confirm payer-specific rules, as Medicaid and commercial payers often differ from CMS.
- Use RPM platforms that integrate with patients’ EMRs to streamline documentation.
- Conduct periodic training for clinical and billing staff.
- Perform mock audits to check the accuracy of clinical documentation.
Challenges to Reimbursement
- Audited claims due to incomplete documentation
- Use of devices not cleared by the FDA
- Misalignment between billing and data collection periods
- Inadequate documentation for interactive communication codes
RPM Billing in Value-Based Care
The increase in value-based care models makes RPM billing worthwhile. Reduced hospitalizations, improved medication adherence, and better chronic disease management are all desired outcomes that align with value-based goals. Many ACOs and health systems have adopted RPM as a key component of their population health strategy.
What Will Happen in 2025?
- Increase oversight and scrutiny to identify and reduce RPM fraud
- Add conditions eligible for reimbursement under RPM
- Shorten the required streak of 16 collected days for certain high-risk patients
- Clarify RPM policy in the behavioral health space
What Are Some Billing “Don’ts”?
- Don’t forget to amend payer contracts for RPM services.
- Don’t use outdated CPT codes.
- Don’t forget to obtain patient consent.
- Don’t forget to track time associated with CPT codes 99457/99458.
CMS Reimbursement Trends (2024–2025)
Approximate national average rates (non-facility settings):
| CPT Code | Avg. Rate (2025) |
|---|---|
| 99453 | $19.38 |
| 99454 | $55.77 |
| 99457 | $50.18 |
| 99458 | $40.84 |
| 99091 | $60–$72 |
Cost Breakdown of RPM Programs
- Device Costs: $25–$150/unit or $15–$30/month (leasing)
- Software Costs: ~$10–$30 per patient/month (PPPM)
- Personnel Costs: 30–60 minutes per patient/month
Provider ROI Considerations
If done well, an RPM program can provide ROI within 3–6 months. A practice with 100+ eligible patients may earn $20,000–$30,000/month in new revenue, depending on staff and software efficiencies.
RPM Reimbursement – Medicaid & Commercial
Commercial Payers:
- Aetna, Humana, and BCBS align with CMS policies.
- UnitedHealthcare uses hybrid RPM + virtual models.
Medicaid Reimbursement (as of 2025):
- 35+ states reimburse for RPM
- Strongest coverage: Texas, California, New York, Colorado
- Some require prior authorization or condition-specific eligibility
Patient Costs
- Medicare patients are responsible for a 20% coinsurance after the deductible unless they have Medigap or supplemental insurance.
- Commercial plans may require cost-sharing unless waived.
Common Reimbursement Challenges with RPM
- Variable private payer coverage
- Denials from lack of documentation
- Staff not familiar with specific billing nuances
Solutions to Improve RPM Reimbursement
- Bundle RPM with CCM or BHI services
- Use daily time-tracking tools for billing (99457/99458)
- Conduct regular audits of your documentation and coding
Future Trends: What Lies Ahead for RPM Software & Devices?

Advancements in technology are expanding RPM capabilities beyond simple data capture and movement towards real-time analytics, predictive care, and personalized engagement. Here are some key trends shaping the future of RPM:
AI-Powered Devices for Predictive Analytics
Next-generation RPM devices will utilize AI to convert raw health data into actionable insights. These systems will be able to recognize early signs of deterioration, assess risk scores, and notify clinicians of potential issues before symptomatology worsens. For example, AI algorithms could look at heart rate variability and predict a potential cardiac event or trends in glucose levels that indicate an impending hypo- or hyperglycemia with high probability.
Smart Inhalers to Manage Asthma and COPD
Smart inhalers with sensors across the inhaler track the medication use, inhaler technique, and frequency of rescue inhaler use. The inhalers transmit real-time data to care teams, and alerts can be sent should the patients’ patterns of use indicate the patient is non-compliant or experiencing poor disease control. Smart inhalers are particularly useful for chronic respiratory diseases, like asthma and chronic obstructive pulmonary disease (COPD), to manage patient’s adherence and reduce hospitalization.
Lightweight Patch Monitors to Support Continuous Biometric Monitoring
Modern patch-based wearables, which require no vests or batteries, can replace heavier Holter monitors. Patch monitors can offer 24 hours of continuous ECG, respiratory rate, skin temperature, and motion tracking but without the heavier apparatus. Ultimately, the goal of using patches is to monitor patients for longer duration (days/weeks), as opposed to a traditional ambulatory diagnostic approach which is partial, and finally, to improve adherence from the patient standpoint by improving comfort/interface.
Voice-enabled RPM Devices
For older adults, visually impaired, or trail-limited mobility patients, getting advice through voice-enabled RPM tools are much easier for those patients to interact with RPM platforms. Patients can use natural language and engage with the RPM platform, ask health-related questions, report symptoms (when more than a yes or no response is wanted), and dispense medication reminders without having to drill down through complex good for touchscreens or cellular app interfaces.
FDA-Approved Wearables with Clinical Use
Leading consumer products such as the Apple Watch, Fitbit Sense, and Samsung Galaxy Watch received FDA clearance as having clinical capacity to use features such as, but not limited to, an electrocardiogram (ECG), atrial fibrillation (AFib) detection, and blood oxygen (SpO₂) features. Consumer wearables represent a new avenue for patients to access remote monitoring by leveraging devices they already own and use in their daily lives, as opposed to lifestyle wearables, providing a continuum of consumer and clinical-grade RPM.
Behavioral Biomarkers in Mental Health Monitoring
RPM’s expansion into mental health is an extension of the how facial expression, sleep, calendar, and smartphone usage behaviors are analyzed. AI-model-driven platforms can recognize and detect early warning signs of symptoms associated with depression, anxiety, or being bipolar. This type of behavioral biometrics creates a low-touch way to monitor mental wellness, and enable early interventions in a pre-symptomatic state.
Remote Patient Monitoring (RPM) Services by HealthArc
HealthArc is a leading digital health platform specializing in Remote Patient Monitoring (RPM), Chronic Care Management (CCM), and Remote Therapeutic Monitoring (RTM) services. HealthArc has developed a comprehensive approach to implement RPM programs so that healthcare providers can positively impact clinical outcomes without increasing their resources and administrative burdens. Some of the key functionalities offered by an RPM platform include:
- Real-time patient monitoring: HealthArc’s RPM platform directly works with and automatically collects vital health data from patients via devices, such as blood pressure, weight, blood glucose, oxygen saturation, and ECG.
- Automated alerts: The HealthArc AI dashboard tracks abnormal trends and alerts our care teams automatically.
- EHR integration: Bi-directional interoperability with leading EHR vendors (Epic, Cerner, Athenahealth) to support documented coordinated care.
- Patient mobile applications: HealthArc provides patients with mobile apps to ensure continued engagement through alerts, reminders, daily logs, and health tips.
- Patient-centered care plans: HealthArc allows providers to customize treatment protocols for care plans based on conditions, such as diabetes, hypertension, CHF, and COPD.
- FDA-approved devices: HealthArc provides a catalog of FDA-approved remote monitoring devices upon shipping to patients, including:
- Bluetooth blood pressure cuffs
- Cellular-enabled weight scales
- Smart glucometers
- Pulse oximeters
- ECG patches and heart monitor patches
All devices are plug-and-play (easy-to-set-up) for patients. Devices are HIPAA-compliant and FDA-compliant in terms of online security.
Provider and Patient Support
- Patient engagement: Multilingual support, device onboarding, training.
- Clinical monitoring: 24-hour clinical teams available to intervene when necessary.
- Billing compliance: Aids providers in navigating and maximizing RPM reimbursement; coding and claim submission are included.
Results and Outcomes
Clients that have been using the HealthArc platform report:
- Hospital readmissions reduced by as much as 50%
- Patient adherence improved by 70% within three months
- Care teams are more efficient
- Uplift in revenue for exploring proper and available billing codes (CPT 99453, 99454, 99457, and 99458)
HealthArc is more than a technology vendor; we are a digital healthcare partner working to improve patient outcomes, reduce clinician burnout, and change the healthcare delivery model through scalable and reliable RPM solutions.
Enhance Patient Outcomes With HealthArc’s RPM Platform
Remote Patient Monitoring has become a present and future reality of proactive, just-in-time care. The many benefits that RPM offers include improved outcomes, decreased costs, sustainable ongoing patient engagement, and empowerment of providers and patients, making it an integral part of modern-day healthcare models.
The urgency of value-based healthcare, the emphasis on chronic disease management, and demand for convenient monitoring solutions have made RPM delivery models one of the best choices for delivering personalized care to chronic patients.
RPM is an integral part of the healthcare delivery model, especially for those in the aging population, rural communities, and individuals with chronic conditions. It allows for improved clinical efficiency, broader access to patient data, increased patient engagement, and reduced unplanned hospitalizations. Patients benefit from the convenience of scheduling, ongoing monitoring, early recognition of deterioration, and lessened isolation from their care teams.
HealthArc has truly accelerated to the forefront by providing HIPAA-compliant RPM solutions for small clinics, solo practices, and large health systems. Its end-to-end remote monitoring platform with real-time analytics, device integrations, and automated workflows makes remote care easy, while keeping the reimbursements high for providers.
If you are a healthcare organization, physician, or health tech innovator looking to implement RPM or improve your existing digital health capabilities, now is the time. Keep yourself informed, connected, and engaged in the remote care industry with the latest RPM software. Request a free demo now to learn how HealthArc integrates RPM, CCM, and AI to reduce hospital readmissions and improve health outcomes.
Frequently Asked Questions (FAQs)
Q1. What are the current CMS reimbursement rates for Remote Patient Monitoring (RPM) in 2025?
As of 2025, CMS reimburses RPM services for the following CPT codes:
- 99453 – Setup of device and educate patient, one time: $19.73
- 99454 – Device supply and data transmission, for the month (16+ days): $43.02
- 99457 – First 20 minutes of RPM treatment management: $47.87/month
- 99458 – Each additional 20 minutes: $38.49
These rates promote the convenience of chronic and acute care monitoring when using FDA-approved, connected devices.
Q2. How does Remote Patient Monitoring (RPM) differ from Chronic Care Management (CCM) and Remote Therapeutic Monitoring (RTM)?
- RPM: Used as recorded physiological data (i.e., blood pressure, glucose) through FDA-approved, connected devices, and for a minimum of 16 days a month.
- CCM: Used with patients with two or more chronic conditions to emphasize care coordination and medication management through ongoing communication, not device data.
- RTM: Uses a software application or similar application, and data will reflect adherence to therapy and musculoskeletal status, and is typically used in Physical or Respiratory Therapy. Unlike RPM, the patient self-reports the data for RTM.
Q3. Which patients qualify for RPM under Medicare and Medicaid in 2025?
Medicare and many Medicaid Plans pay for RPM:
- Patients with chronic or acute collections requiring monitoring;
- FDA-approved devices, used in connection with the automatic transmission of information;
- Data tracked for a minimum of 16 days in 30 days; and,
- NOTE: State regulations on Medicaid change; therefore, providers have to check state rules.
Q4. How are RPM devices secured and HIPAA-compliant?
Devices and platforms used for RPM must comply with HIPAA requirements as follows:
- Encrypted during transmission end-to-end
- Encrypted data storage that is stored encrypted (storage encryption)
- Use of access controls, audit logs, and role-based permissions to protect patient information
- RPM vendors generally use cloud-based, HIPAA-compliant platforms that secure patient privacy and security at all relevant points.
Q5. Can RPM platforms detect early deterioration with AI algorithms?
Yes, contemporary RPM platforms leverage artificial intelligence and predictive analytics to identify early signs of clinical deterioration by analyzing trends within vital signs and physiologic data. These trends enable providers to initiate a proactive intervention prior to the patient visiting an emergency room.
Q6. What interoperability standards (HL7, FHIR, API) do modern RPM platforms support?
Platforms used for RPM will incorporate interoperability standards including (but not limited to) Fast Healthcare Interoperability Resources (FHIR), HL7, and RESTful APIs to ensure seamless integration into EHR systems as it relates to data exchange and coordination of care.
Q7. How does RPM improve health equity in rural and underserved populations?
RPM provides the opportunity to circumvent access barriers to healthcare by providing remote monitoring, thus alleviating travel concerns, encumbering continuity of care and access to healthcare, especially for patients in rural settings and geographically underserved areas, thereby closing the access gap to improve health equity and outcomes.
Q8. What documentation is required for CPT code 99457 billing?
Providers intending to bill CTP 99457 must document:
- Patient consent (verbal or written)
- Person providing real-time interaction (≥20 minutes/month by clinical staff or provider)
- Treatment management decisions involving the RPM data
- Date, duration, and content of the service
Q9. How often must RPM data be collected to qualify for Medicare billing?
To receive reimbursement from Medicare for CPT 99454, RPM data must be collected and transmitted for 16 days or more in 30 days using an FDA-designated connected device.
Q10. What are common barriers to RPM implementation, and how can they be addressed?
Key barriers to RPM are lack of digital literacy, lack of broadband access, difficulty integrating RPM into workflow, and provider buy-in. To overcome barriers, patients need RPM education, easy-to-use devices, integration with the EMR, and staff training in RPM protocols.